|
Friedland Assessment and Strengthening Tools for Speech Therapy |
||||||||||||||||||||||||||||||||||||
|
Contact Information FAST Speech Therapy 1280 Route 46 W., Parsippany, NJ 07054 (973) 334-3000 (201) 247-4419 Management Team Eric Friedland, MS CCC SLP, SLS (Chief Medical Officer) Mark Annett, MS BMe, (CTO/Patent Agent – 69401) Industry Medical Devices, Speech Therapy Company Resources Friedland Assessment and Strengthening Tools for Therapy (FAST Speech Therapy) is a venture that has access to the resources of United Speech Services as well access to the engineering resources of Annett Enterprises LLC. Type of Financing Sought: $1.5M seed Pre-Money Valuation for this Round: TBD Total External Capital Invested -NA- Professionals IP Legal – Weinick, Jeffrey M. WOLFF & SAMSON PC One Boland Drive West Orange, NJ 07052 (973) 530-2028 Accounting firm - TBD Corporate Legal - TBD Bank - TBD Use of Funds Mold qualification, initial production, clinical trials, marketing and salaries Year Founded 2009 Type of Entity Planned as LLC |
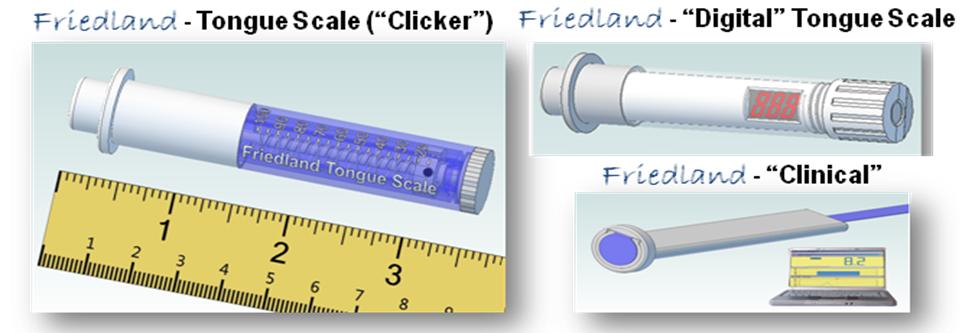
Business Description The mission of Friedland Assessment and Strengthening Tools for Speech Therapy (also known as FAST Speech Therapy) is to develop therapeutic tools that will not only be available in specialty clinics but are broadly available in all settings, including the patient's home. Our commitment is to develop best in class tools for assessment and strengthening of speech and swallowing skills that are able to achieve documented improvement in patients, and are affordable by both clinicians and the patients themselves. Management Chief Medical Officer - Eric Friedland, MS CCC SLP, SLS – Eric is a Medical Speech Language Pathologist with over 30 years experience in all clinical settings from schools to geriatric facilities. He is the administrator and owner for the past 17 years of United Speech Services and is certified in providing Fiber Endoscopic Evaluation of Swallowing (FEES and FEEST) and VitalStim. Chief Technology Officer - Mark Annett, MS BMe, Patent Agent #69401 – Mark is a bio-medical engineer/patent agent with 25+ years experience in all phases of medical devices, from design and IP, through regulatory submissions and vendor qualifications, and into the market and beyond. Company Background The first US Patent Application 13/013,001, was created In response to the need to quantify tongue strength for a more complete oral musculature examination. Speech Pathologists and other medical professionals will be able to quantify tongue strength utilizing a standardized scale. Measurement of tongue strength is important for swallowing function or to assess unilateral or bilateral tongue weakness as presented in a stroke. Therapy is accomplished by strength against resistance exercises. This tongue measurement system is applicable for children and adults. The response from peers and colleagues has been favorable. This led us to the development of a family of products known as the Friedland - Tongue Scale System, which allowed us to achieve our vision of creating not only world class speech products that are affordable by both clinicians and their patients but will be manufactured here in the US. Technology/Proprietary Rights Initial launch will be with a family of products called, the Friedland - Tongue Scale System (see below). The family of products is patent pending per US Application number: 13/307,459 and per International Application number: PCT/US2011/062629. Additional intellectual property includes US Application 13/013,001.
Marketing, Sales and Customers TOTAL Domestic market was established by taking a portion of the estimated 153,700 speech therapists in the U.S. as specified in the National Employment Matrix for 2018 and the size of the TOTAL International market was projected to be 200% of the Domestic. Sales would be through two channels: direct marketing to clinicians through continuing education, tradeshows, conferences, and publications AND directly to the patients themselves through our website www.FASTSpeechTherapy.com. Competition Although there have been several patents issued for tongue strength measurement devices, only one device, US Patent Number 5,119,381 (June 9, 1982), is currently on the market, as the Oral Performance Instrument (IOPI), www.iopimedical.com. This device only competes with our clinical version and since it is off-patent we would anticipate an equivalent product as a component of our clinical version. A new product that is coming out shortly is the MADISON ORAL STRENGTHENING THERAPEUTIC. (MOST®) DEVICE. by Swallow Solutions LLC www.swallowsolutions.com, again, this product only competes with our clinical version. |
|||||||||||||||||||||||||||||||||||
|
Financial Results and Projections (dollars in millions)
|
||||||||||||||||||||||||||||||||||||